New synthetic polymers show promise for creating rugged, environmentally resilient medicines that don’t lose potency over time
Soldiers often operate in extreme environments, where they may be exposed to the elements for long periods of time. Standard equipment such as electronics and armor are designed to withstand such stresses, but that is not true for the contents of a medic’s bag. Most medicines, including essential biotherapeutics such as insulin, degrade rapidly when stored outside of specified temperature, humidity, and light conditions. DARPA’s Fold F(x) program aims to develop new classes of rugged, shelf-stable medicines based on non-natural, synthetic polymers that can better withstand extreme conditions in the field. The Agency will hold a workshop next week to describe recent successes and discuss applications with potential collaborators.
Biopolymers are large molecules created by stringing together smaller biochemical units, called monomers. Proteins are biopolymers made of hundreds or thousands of monomers called amino acids. The specific amino acids that are strung together to make a protein, and their linear order or “sequence,” define the protein’s final three-dimensional shape and, by virtue of that shape, its function. For example, insulin binds to specific molecular structures on the surface of cells precisely because of its specific 3D (folded) shape, which is determined by the sequence of its amino acid building blocks. Performers in the Fold F(x) program are leveraging this concept of “sequence defines shape defines function” to mimic the activity of naturally occurring biopolymers using completely new, laboratory-produced monomers. Because these monomers are synthetic, they can be designed to be more robust than their natural amino acid counterparts, opening the door to making proteins with new functional properties.
Since the late 1960s, researchers worldwide have attempted to design specific non-natural polymer sequences that would fold into specific three-dimensional shapes and perform a desired medicinal function. These one-off, targeted attempts at “rational drug design” have not yielded much success, in large part because scientists still have only a rudimentary means of predicting how the multitude of monomers in a biopolymer will interact and fold on one another. Performers in the Fold F(x) program are taking a completely different approach. Instead of trying to create the one perfectly folded polymer that will accomplish a desired biomedical function, they are creating massive suites of non-natural polymers with at least a billion distinct sequences that are hypothesized to fold into some generally desirable class of 3D shapes. They are then screening these polymer libraries with cutting-edge sorting technologies to identify which sequences in each library bind to a particular target of interest. The approach mimics that of the human immune system, which does not instantly produce antibodies that bind specifically to a newly encountered pathogen but rather produces a shotgun blast of varied antibodies, then amplifies production of the antibody that proves most effective at binding to and disabling the attacking microbe.
There are significant challenges to producing large libraries of biopolymers and to screening for those best suited to a desired task, but the Fold F(x) program has been enjoying a string of successes. To date, six performer groups have built billion-plus non-natural polymer libraries and identified members that bind to known targets in application areas ranging from biopharmaceutical production to biowarfare agent detection. One group at the Massachusetts Institute of Technology (MIT), for example, created a non-natural polymer with demonstrated thermal and environmental stability that can recognize the well-known biowarfare agent anthrax. That specific recognition may be exploited to develop a rapid, field-stable diagnostic test for anthrax.
MIT is now also screening its non-natural polymer library in collaboration with the U.S. Army Medical Research Institute of Infectious Diseases to create synthetic biopolymers that bind to Ebola, in the hope of creating a totally new and robust Ebola treatment. A separate effort at Stanford University will use a newly developed screening capability based on high-throughput imaging technology to identify non-natural polymers that bind to inactivated Burkholderia pseudomallei and Burkholderia mallei, two bacterial pathogens of significant interest to the DoD.
Other teams have used the billion-member library strategy of the Fold F(x) program to generate and rapidly identify synthetic biopolymers that interact in very specific ways with a number of other targets. Some biopolymers are predicted to be resistant to digestion, for example, opening the door to oral versions of medicines that today must be injected because they would otherwise break down in the stomach. Others show promise as temperature-resilient compounds that could remain potent for decades instead of years, or shelf-stable diagnostics that could someday replace current counterparts that need to be kept refrigerated. Collaborators in DARPA’s Fold F(x) program include University of California at Irvine, Harvard University, Scripps Research Institute, Lawrence Berkeley National Laboratory, and SRI International.
“With impressive results like these and ambitious new projects already underway, Fold F(x) is on track to improve biotherapeutic treatments and diagnostics, with potential benefits not just for our soldiers in combat but also for a wide array of civilian and commercial medical challenges,” said Tyler McQuade, DARPA program manager. “Just as plastics, which are non-biological polymers, transformed manufacturing in the middle of the last century, I see biopolymers revolutionizing biology and medicine in the decades ahead.”
The Fold F(x) program is positioned to build on its initial successes by generating, screening, and testing ever larger numbers of non-natural polymers that specifically bind to targets of high relevance to both the DoD and the civilian public health sector. Both diagnostic and therapeutic applications are envisioned—not only for bacterial and viral pathogens but also for toxins and resilience-relevant host-response targets.
DARPA will host a workshop on June 29, 2016, in Southbridge, Massachusetts, at which DARPA-funded Fold F(x) researchers will share with military and civilian public-sector stakeholders as well as with venture firms and potential private-sector collaborators details about the role Fold F(x) non-natural polymers could play in the future of biomedicine. Registration is required for workshop attendance, and space is limited. Interested parties should contact Dr. McQuade at https://go.usa.gov/cJJxQ.
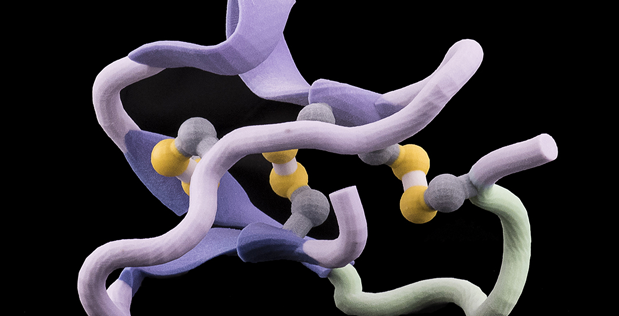
Image Caption: A 3-D printed model of a non-natural Xenoprotein Anthrax Binder developed under DARPA’s Fold F(x) program by researchers at MIT. This particular folded shape binds to the Anthrax pathogen, which is of interest to DoD. (DARPA Photo)